COVID-19 pandemic has put healthcare systems of various countries, including Indonesia, to the test. Many problems that were previously unnoticed are now gaining attention. One of them is Indonesia’s lack of readiness to detect the SARS-CoV-2 virus—commonly known as COVID-19—in order to prevent its spread and speed up treatment for the infected individuals. In the context of Southeast Asian countries, Indonesia’s COVID-19 testing capacity (1.03 tests per 1,000 inhabitants) is among the lowest. In comparison, the testing capacities of the Philippines, Thailand, and Malaysia reach 3.71, 6.7, and 19.17 tests per 1,000 inhabitants respectively.
Based on the online media news we monitored from January to June 2020, the frequently mentioned causes are the lack of laboratory personnel and testing kits for the polymerase chain reaction (PCR) method. Furthermore, the constantly changing and ineffective policies show that the government is not prepared in tackling the pandemic. Strengthening coordination between the central government and regional governments, as well as between the government and the private sector, must be prioritized to expedite COVID-19 testing in Indonesia. It is also necessary for the government to develop an integrated data system that is always updated so that the needs for analysts and laboratory equipment can be monitored continuously.
Testing Capacity Improvement Has Yet to Meet the Target
Since COVID-19 became worldwide at the end of January 2020, the Government of Indonesia’s preparedness in preventing its spread has been under the spotlight. With a population of 270 million spread across numerous islands, Indonesia should anticipate the spread of COVID-19 more seriously. Close monitoring measures are needed because Indonesia has around 135 entrances by land, sea, and air and receives thousands of visitors from abroad every day. Even though the government had initially stated that they were ready to face COVID-19, they did not support the statement by making capacity building efforts in order that provinces and kabupaten/kota (districts/cities) can detect the coronavirus.
"With sufficient PCR tools, we can analyze more than 1,000 samples in a day. To the Indonesian public and other countries that have doubted Indonesia’s capability, I can assure you that we are capable." (Coordinating Minister for Human Development and Culture, Muhadjir Effendy, kumparan.com, 13 February 2020)
On the other hand, decentralization has caused provincial and kabupaten/kota governments to have different capabilities in dealing with health issues, including coronavirus detection. It is unfortunate that the initiatives of several regional heads, who felt that their regions could provide PCR tests, were rejected by the Minister of Health by reason of preventing intervention by certain parties.
"I am sure many are questioning my policy of why I am using only one laboratory. The key is, no laboratories should be influenced by parties with a vested interest." (Minister of Health, Terawan Agus Putranto, www.suara.com, 29 February 2020)
When early COVID-19 cases were detected in early March 2020, the government’s ineptitude, especially regarding testing, started to come into view. The government’s initial plan to centralize PCR tests at the Health and Research Development Agency (Balitbangkes) was changed several times as the number of COVID-19 cases were rising rapidly in various regions. On 3 March 2020, a day after President Joko Widodo announced two positive cases, the Ministry of Health issued Decree of the Minister of Health No. HK.01.07/MENKES/182/2020 on COVID-19 Testing Laboratory Network. The decree stipulates that the Ministry of Health has added ten laboratories, spread across some provinces, into the COVID-19 testing laboratory network. It also mentions that the laboratories must still send their specimens to Balitbangkes for confirmation. Furthermore, announcements about positive cases in every region must be made by Balitbangkes and the Ministry of Health. On 19 March 2020, the number of testing laboratories significantly increased to 48. Also, the line of reporting was no longer centralized at Balitbangkes. Nevertheless, the number of specimens tested per day did not experience a significant increase (Figure 1). At the end of March 2020, the government also attempted to coordinate with private laboratories to increase the number of PCR testing laboratories, but there were no immediate results.
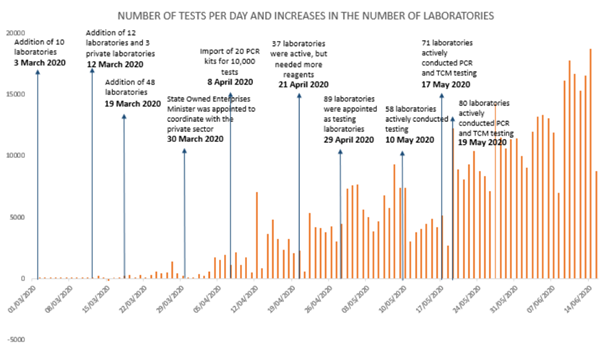
Figure 1. Number of tests per day in Indonesia and the policy timeline
Source: BNPB.
President Joko Widodo had set a target of 10,000-specimen tests by April 2020 and 20,000-specimen tests by June 2020. The 10,000-test-per-day target was driven by the government’s success in importing 20 PCR kits, which were claimed to be effectual for conducting up to 10,000 tests per day. Furthermore, Indonesia increased the number of laboratories for molecular rapid test (TCM), though not all of them were active yet.
Several hurdles had caused the government to miss the target. It was only in late May 2020 that the number of tests could reach 12,000 in a day. In early June, the President optimistically increased the testing target to 20,000 specimens per day. However, on 8 June 2020, the number of tests dropped sharply to 6,988. The government said that the lack of support for testing, such as laboratory personnel, reagents, and viral transport media (VTM), caused the coronavirus testing to slow down in several regions.
“So far, the challenge is the lack of human resources in every laboratory. Personnel is still limited, while reagents and test kits are already sufficient.” (Head of COVID-19 Response Acceleration Task Force, Doni Monardo, nasional.tempo.co, 4 May 2020)
“Some of the existing laboratories can no longer conduct testing and the reagents have not yet arrived. However, most of them can already conduct testing, and today, we help to add the reagents.” (Government’s COVID-19 Handling spokesperson, Achmad Yurianto, www.voaindonesia.com, 2 May 2020)
Another challenge in the procurement of laboratories for PCR testing is the coordination between the central and regional governments, as well as the government and the private sector.
Coordination Problem: Central Government, Regional Governments, and Private Sector
The constantly changing regulations made by the Ministry of Health in only a short time shows that the government was responsive and flexible in addressing the needs on the field. However, it also shows that the government was not prepared to handle this pandemic. Furthermore, adding more laboratories and changing the line of reporting did not immediately increase the testing capacity of every laboratory (Figure 1). In the time span from the COVID-19 starting to spread worldwide (late January 2020) to the COVID-19 entering Indonesia for the first time (March 2020), the government actually had enough time to take preparation measures, such as strengthening coordination between the central and regional governments, to increase testing and hospital capacities, as well as accelerate cooperation with the private sector to improve detection.
The lack of coordination between the central and regional governments was also visible from the process of appointing regional laboratories as testing laboratories. The readiness of a region should have been ensured first before a laboratory in the region was appointed as a testing laboratory. However, in reality, the decree on the addition of testing laboratories seemed like a false hope because registered laboratories could not operate immediately. Moreover, there came an impression that the appointment of laboratories, which was conducted by the Ministry of Health, did not involve coordination with the regional heads.
Based on the Decree of Minister of Health No. HK.01.07/MENKES/214/2020, the laboratories that are appointed as testing laboratories must fulfill the biosafety level 2 (BSL-2) standard and have real-time PCR testing kits. Furthermore, point 11 of the decree states, “Minister of Health can appoint COVID-19 testing laboratories besides those that have been appointed in this Decree, after going through verification on COVID-19 testing capability.” From these two provisions, there are several things that we can criticize.
First, there are no provisions that require the readiness of personnel and supporting tools for testing with Real-Time PCR for laboratories that are appointed as testing laboratories. It is not surprising that some appointed laboratories in some regions seemed unprepared. The Aceh Balitbangkes is one of the examples. This laboratory started operating on 16 April 2020, almost a month after its appointment on 19 March 2020. This happened because the Aceh Balitbangkes still had to improve its laboratory’s condition to adjust to the security and health protocols set by the central government and the World Health Organization (WHO). Similarly, the Dr. Kariadi Hospital in Semarang also could not start operating even though it had been appointed.
"In Central Java, the appointed institutions are B2P2VRP Salatiga and Dr. Kariadi Hospital in Semarang, but the one operating in Salatiga was specifically built for that. Dr. Kariadi Hospital still needs some push to be prepared." (Central Java Governor, Ganjar Pranowo, jateng.antaranews.com, 26 Maret 2020)
Second, a strict provision, which is the verification of COVID-19 testing capability, is applied to laboratories that will be appointed later (not to the main laboratories that have been appointed before). At this point, a big question emerges: why aren’t the verification requirements set from the beginning for all laboratories to be appointed as testing laboratories?
A lack of coordination between the central and regional governments regarding testing was also seen in the early reporting of COVID-19 cases. Several regions initially disseminated by themselves the increase in COVID-19 cases or deaths of COVID-19 suspects. This caused discrepancies in the data announced by the central government and the data announced by regional governments. In response to this, the central government stressed that the announcement of coronavirus infection cases should only be done through a one-door channel.
"We will coordinate this matter at the regional level. Once again, let medical experts handle medical matters. Do not let others handle them because there would be biases." (Government’s COVID-19 Handling spokesperson, Achmad Yurianto, nasional.kompas.com, 3 March 2020)
This occurrence shows disharmony between the central and regional governments and ineffective dissemination of line of reporting from the central government to regional governments. On the other hand, this problem is deemed hampering regional governments to provide immediate responses to COVID-19 cases in their regions.
“The spread is fast, so why are information and mitigation being obstructed? This prevents us from anticipating it on time.” (Trisakti University Public Policy Lecturer, Trubus Rahardiansah, tirto.id, 14 March 2020)
The coordination between the central government and the private sector must also be scrutinized. Based on the online media we monitored, the news about coordination between the government and the private sector was mostly published on 30–31 March 2020. At the time, the government announced that COVID-19 testing by the private sector was under the coordination of the Ministry of State-Owned Enterprises. This decision was made to respond to, among others, the slow improvement in testing capacity of the laboratories managed by the government.
"Given the limited capacities of laboratories and research institutions at the regional level, the right solution is to involve the private sector." (Head of COVID-19 Task Force, Doni Monardo, www.vivanews.com, 30 March 2020)
From the online media news we monitored, there can be seen an increase in the number of hospitals or private laboratories and university laboratories that are working with the government. However, it seems that an ideal form of cooperation has not been agreed on. The coronavirus testing still has not achieved the target because laboratories at hospitals and universities have days off. The government should provide incentives or additional personnel for coronavirus testing by the private sector so that it can be conducted 24 hours to expedite the detection of COVID-19 spread.
"We have put in our maximum efforts so that all laboratories will not stop operating even though it is holiday. However, several laboratories at hospitals and universities still apply holidays." (Government’s COVID-19 Handling spokesperson, Achmad Yurianto, www.solopos.com, 15 June 2020)
Coordination problems from the government’s side culminated in several appointed laboratories not operating yet. The delay in imports and slow distribution of testing kits also caused specimen testing to stop. Nevertheless, the government also provided appropriate responses by developing and producing PCR test kits for domestic needs.
Lack of PCR Test Kits
Another factor that affects the limited PCR testing capacity in Indonesia is the lack of PCR test kits. Until early May 2020, there were still many regions that reported a delay in COVID-19 detection due to the lack of reagents and VTM. Unlike South Korea that produces its own test kits, Indonesia still has to import all COVID-19 detection kits. Furthermore, the Government of Indonesia must compete with other countries to obtain those kits because almost all countries need them. It also takes time to distribute the kits to various regions—this will make the 10,000-tests-per-day target even longer to be achieved.
“To date, from the existing laboratories, there are some that can no longer conduct testing as the reagents have not arrived. However, most of them can already conduct testing and today, we already helped to add more reagents.” (Government’s COVID-19 Handling spokesperson, Achmad Yurianto, www.voaindonesia.com, 3 May 2020)
Even though there was a delay in the arrival of imported reagents, which led to the postponement of COVID-19 testing, the swift work of the COVID-19 Task Force in fixing the import problem and distributing PCR test kits needs to be appreciated. On 4 June 2020, the Head of COVID-19 Task Force stated that the cooperation with other countries had improved, meaning that the supply of reagents and other needs should be of no concern in the future. Our monitoring of online media reporting in June 2020 did not discover any more news about the lack of test kits at the regional level. Also, there was no more negative news about coordination between the central and regional governments in providing COVID-19 test kits.
"Whenever we need reagents, we can bring in the supplies. Several elements of the private sector have also tried to obtain reagents, so that we can combine them later.” (Head of COVID-19 Task Force, Doni Monardo, nasional.tempo.co, 4 June 2020)
Since March 2020, besides ensuring enough imported PCR test kits, the government has also focused on developing domestically produced PCR test kits and mobile PCR. The three-month technological development was led by the Agency for the Assessment and Application of Technology (BPPT). The speed of the research up until production is a breath of fresh air for efforts to improve testing capacity in Indonesia. Moreover, the development of Indonesia’s medical equipment industry could be considered slow and heavily dependent on other countries. On 20 May 2020, 50,000 test kits were successfully produced and distributed to 16 regions in Indonesia.
Lack of Laboratory Personnel
While PCR test kits are increasingly available, increasing the number of human resources for laboratory testing has not found a solution. The lack of personnel to test COVID-19 specimens is one of the root causes of the limited COVID-19 testing in Indonesia. This lack of human resources has caused a situation that only a few laboratories can operate 24 hours a day to conduct the testing. From early April 2020 to June 2020, extending laboratory operating hours became the focus of the government in order to achieve the target of 20,000 tests per day.
"The number of laboratory personnel is limited. They are expected to work for 24 hours, but they now can only work for 8 hours. If possible, the number of human resources should be increased through the Indonesian Medical Association’s help, so that the working hours could be increased to 16 hours." Head of COVID-19 Response Acceleration Task Force, Doni Monardo, republika.co.id, 4 June 2020)
Even though the main idea in practice is the same, which is volunteer recruitment, there is not yet synergy among institutions to increase the number of laboratory personnel. For instance, on 8 May 2020, Indonesian Institute of Sciences (LIPI) trained 600 volunteers from laboratories, hospitals, and universities. Meanwhile, the Ministry of health had trained 300 medical volunteers to help with COVID-19 testing. The Coordinating Minister for Human Development and Culture also mentioned the urgency of recruiting volunteers, especially from master’s students in health science, for specimen testing.
Despite the training idea starting to be practiced, the number of needed laboratory assistants for COVID-10 testing has not been clearly identified. Up until late May 2020, the COVID-19 Task Force was still looking for a way and keeping communications at the regional level about the number of personnel needed. This complication occurred because there was no integrated system to collect data on the lack of human resources in each region. The development of this kind of system is important for the planning and distribution of technical personnel. With this integrated system, policymakers could identify which region still needs more resources and focus on recruiting and training volunteers in each region.
Still about the volunteers, the need for a laboratory assistance position should be standardized so that the recruitment is effective. LIPI, the Ministry of Health, and the Coordinating Minister for Human Development and Culture seem to set different basic experience and education requirements for volunteers. The Minister’s idea to recruit volunteers from master’s students should be questioned. A large number of volunteers are needed to accelerate COVID-19 testing while the number of master’s students in health science in Indonesia is limited. The volunteer recruitment should also target bachelor’s students who are in their final year or have graduated but not found a job yet.
“Machines need operators. We must recruit personnel who are still at school. There are many school or private laboratories, but they have to be trained first.” (University of Indonesia’s epidemiologist, Dr. Pandu Riono, today.line.me, 5 May 2020)
Improvement for the Future
COVID-19 testing capacity in Indonesia has increased steadily even though the main target has not been achieved consistently. The problems in PCR testing emerged more at the beginning of the COVID-19 pandemic due to the government’s lack of preparation in handling the pandemic. However, it is still possible that miscommunications and lack of coordination in COVID-19 testing could happen again in the future, such as during the new normal or the second wave of the COVID-19 pandemic. In order to ensure that the testing capacity will improve, there are several things that need to be done:
- ensure coordination between the central and regional governments through regular communication and dissemination of policies by both parties;
- provide incentives and additional personnel for government and private laboratories so that they can operate 24 hours per day;
- establish volunteer recruitment standards for PCR-testing job; and
- develop an integrated system for PCR test resources in terms of the PCR test kits and personnel at the provincial level. Again, coordination and communication between the central and regional governments are crucial in ensuring that this system can run properly.